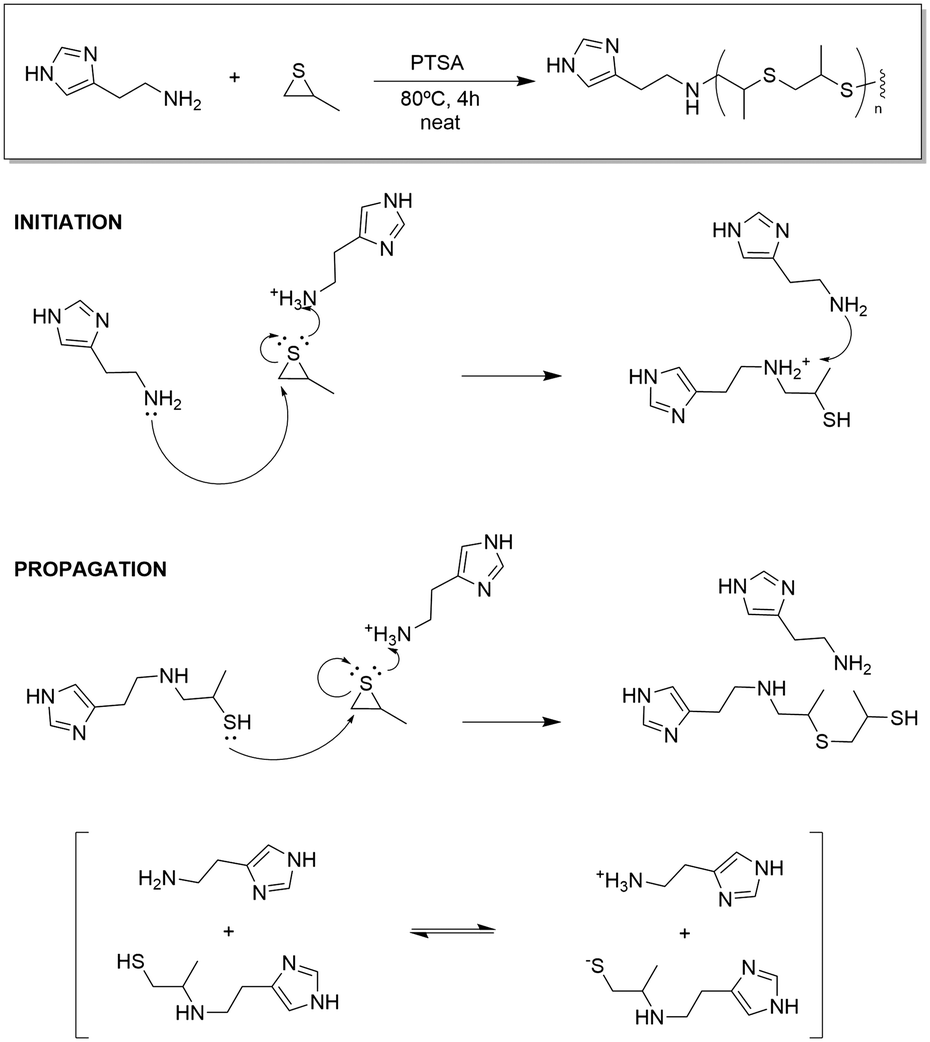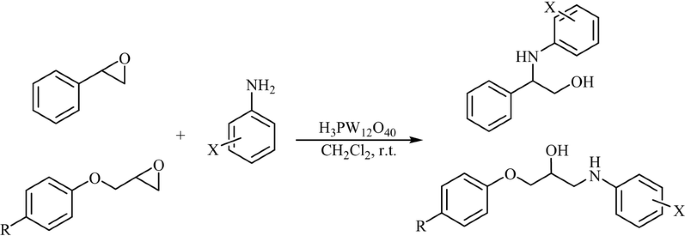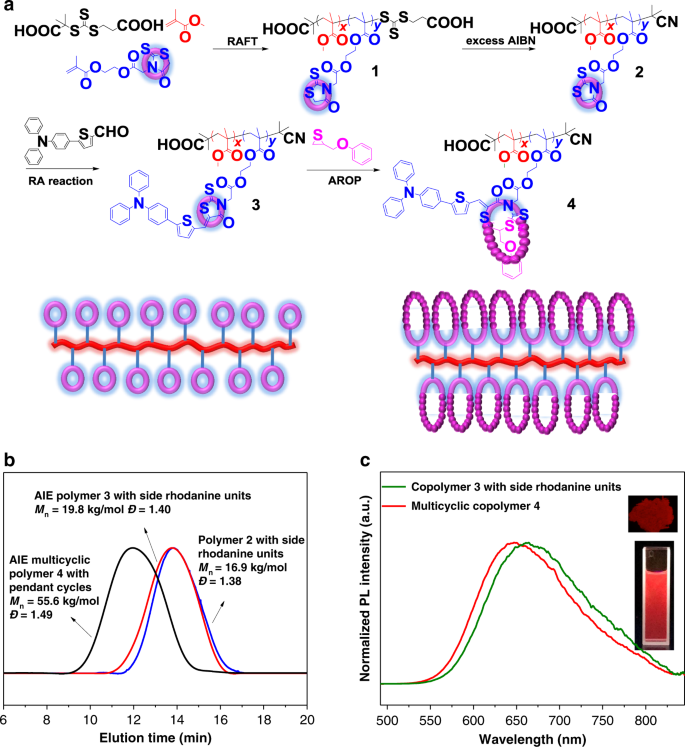
Rhodanine-based Knoevenagel reaction and ring-opening polymerization for efficiently constructing multicyclic polymers | Nature Communications

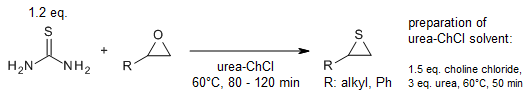
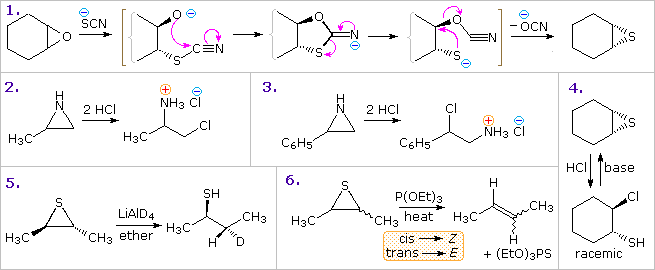
LiAlH4‐Induced Thia‐Aza‐Payne Rearrangement of Functionalized 2‐(Thiocyanatomethyl)aziridines into 2‐(Aminomethyl)thiiranes as an Entry to 5‐(Chloromethyl)thiazolidin‐2‐ones - Dolfen - 2017 - European Journal of Organic Chemistry - Wiley Online Library

Rhodanine-based Knoevenagel reaction and ring-opening polymerization for efficiently constructing multicyclic polymers | Nature Communications
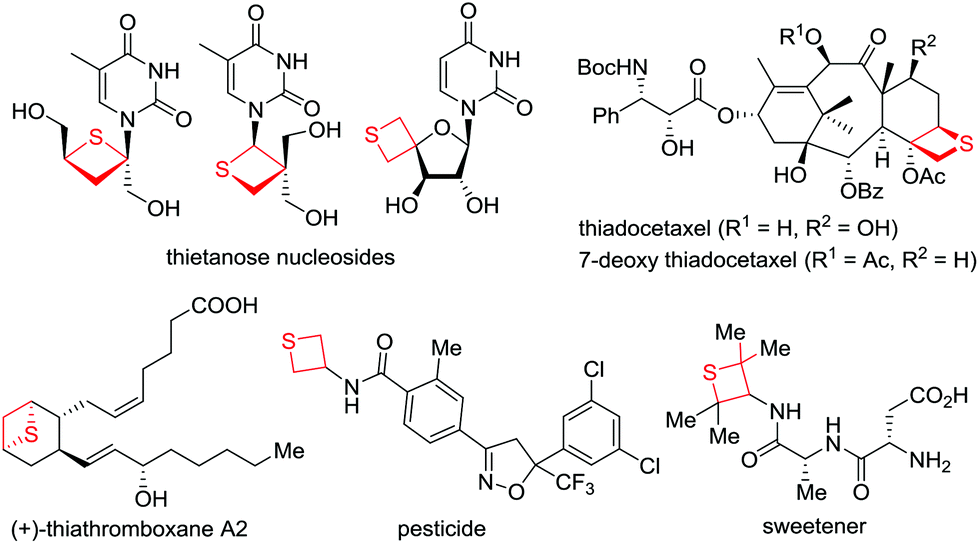
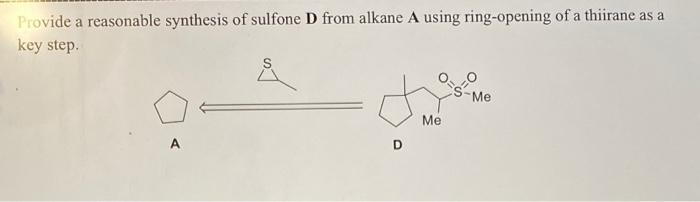
Facile synthesis of thietanes via ring expansion of thiiranes - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C6OB02387H
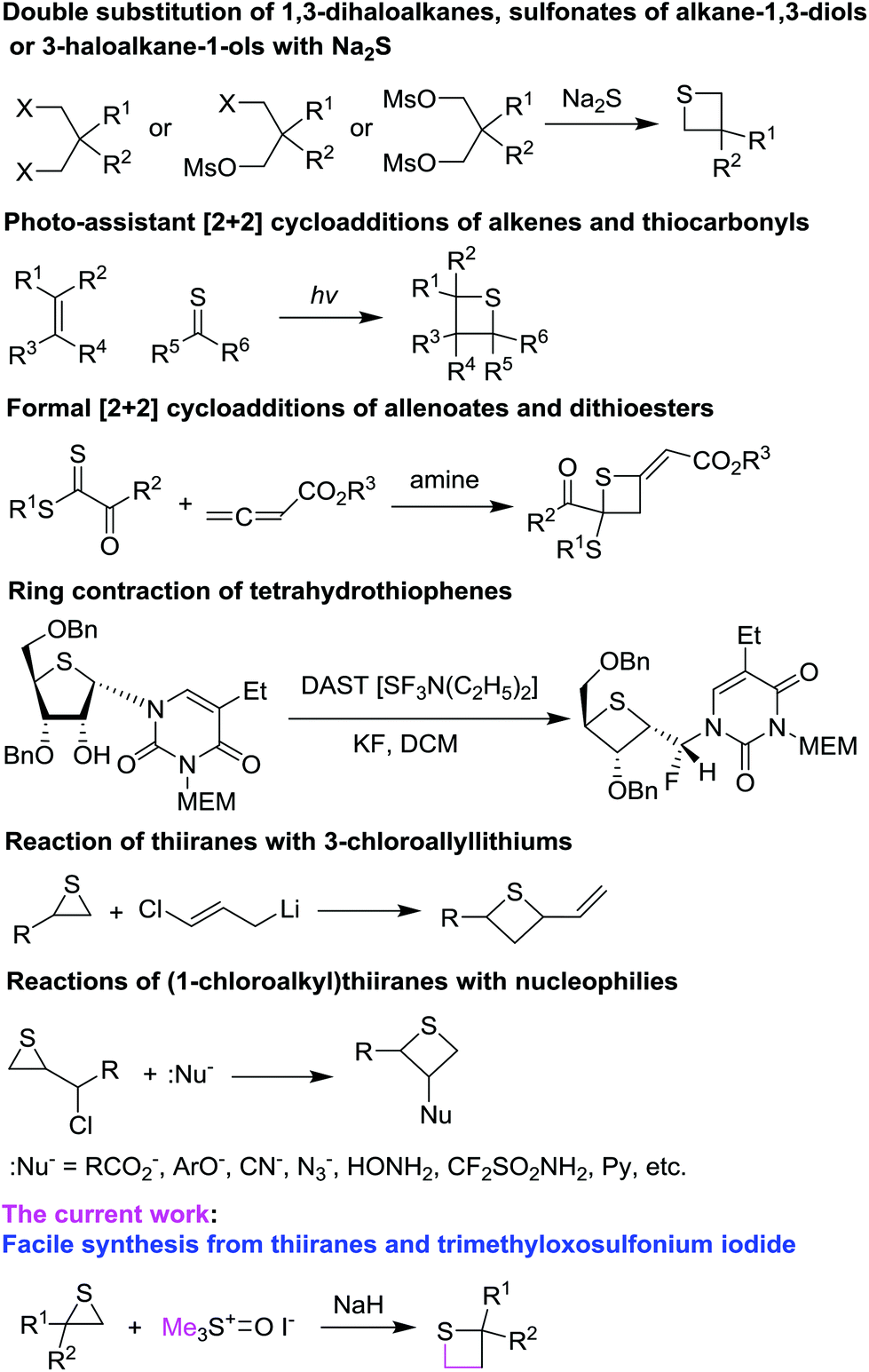
Facile synthesis of thietanes via ring expansion of thiiranes - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C6OB02387H
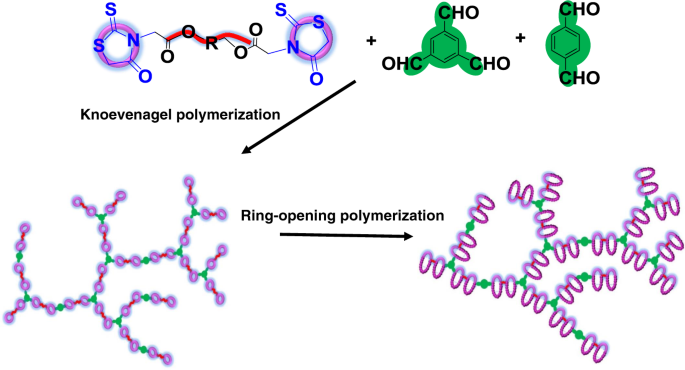
Rhodanine-based Knoevenagel reaction and ring-opening polymerization for efficiently constructing multicyclic polymers | Nature Communications

Controlled Radical Polymerization of 2,3‐Epithiopropyl Methacrylate - Tebaldi de Sordi - 2007 - Macromolecular Rapid Communications - Wiley Online Library

Ring opening polymerization and ring opening copolymerization catalyzed... | Download Scientific Diagram

Bis(trimethylsilyl)selenide in the Selective Synthesis of β‐Hydroxy, β‐Mercapto, and β‐Amino Diorganyl Diselenides and Selenides Through Ring Opening of Strained Heterocycles - Tanini - 2015 - European Journal of Organic Chemistry - Wiley

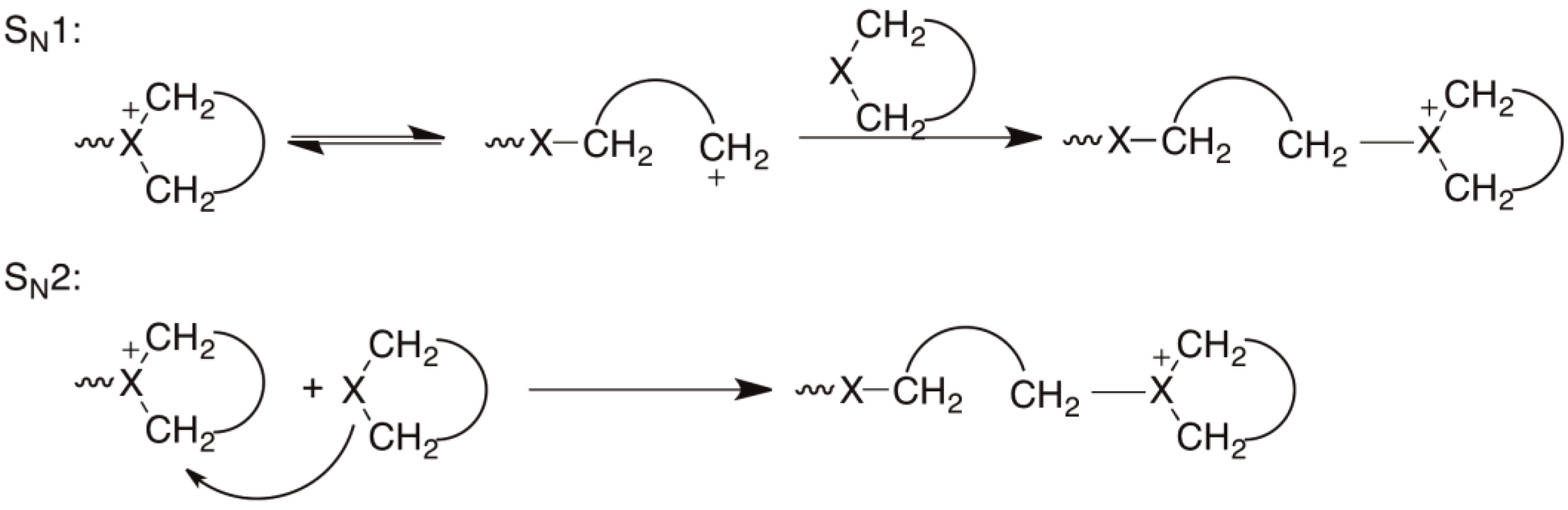
organic chemistry - Will a Grignard reagent react with an epoxide or episulfide first? - Chemistry Stack Exchange

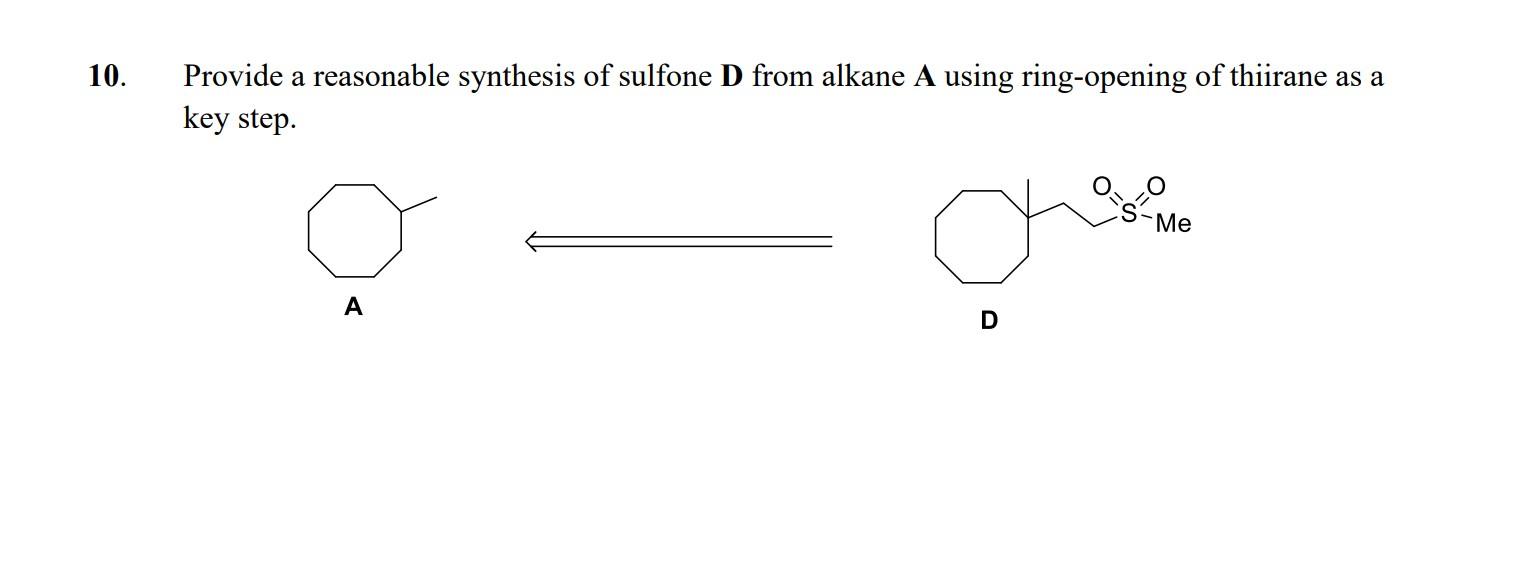
Synthesis of Four- to Seven-Membered Heterocycles by Ring Expansion: Ring Expansions of Thiiranes and Thietanes | SpringerLink

Theoretical calculational investigation on the regioselectivity of the ring opening of thiiranes with ammonia and amines - ScienceDirect