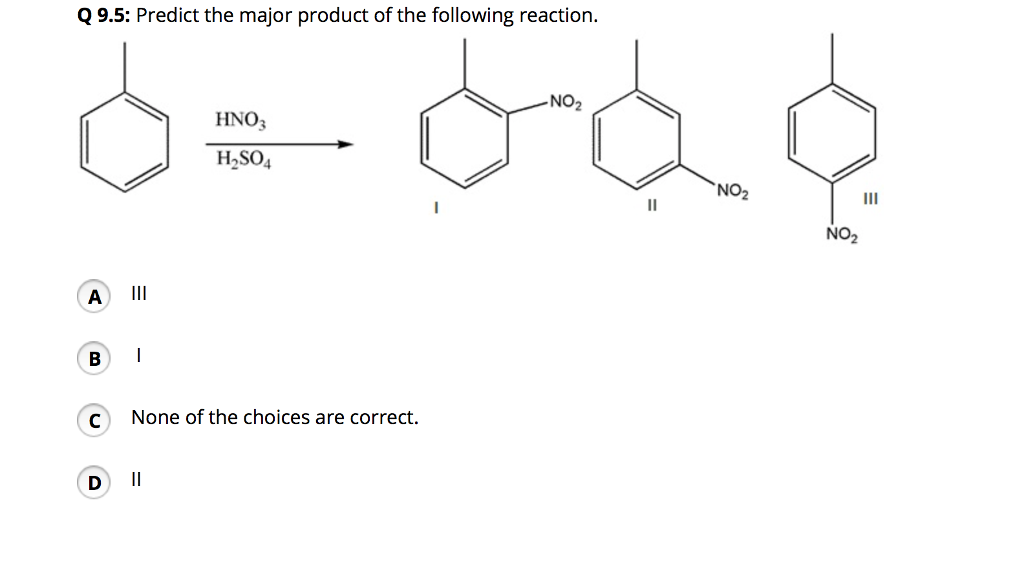
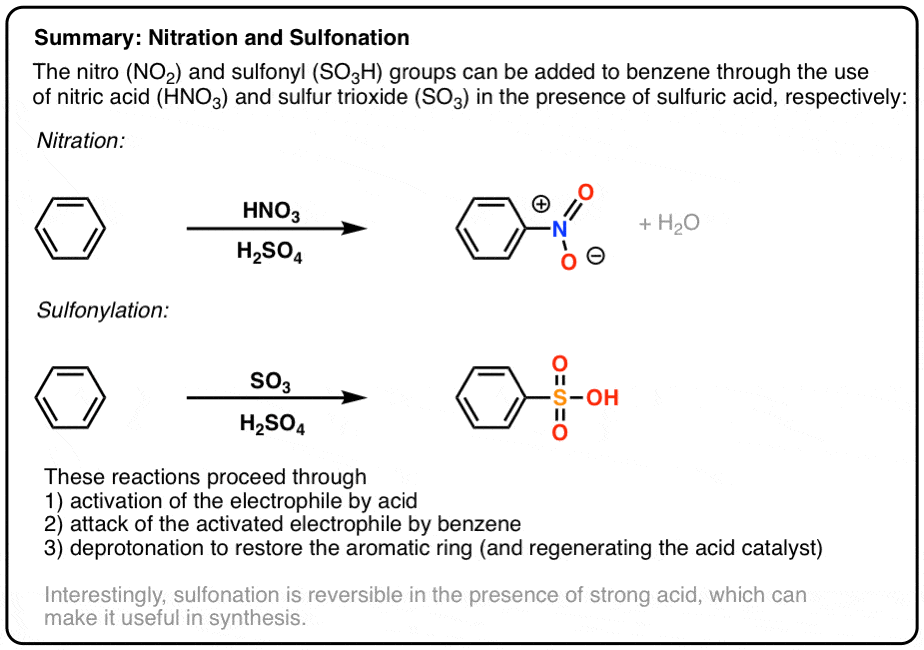
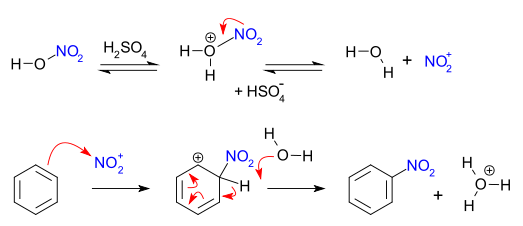
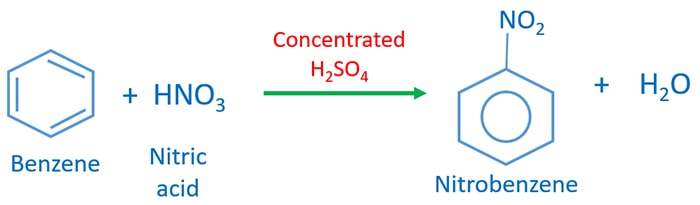
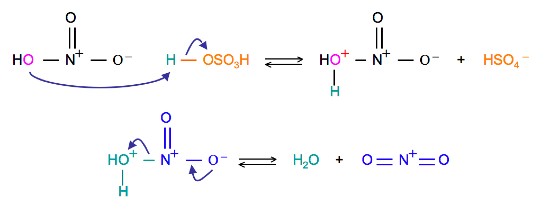
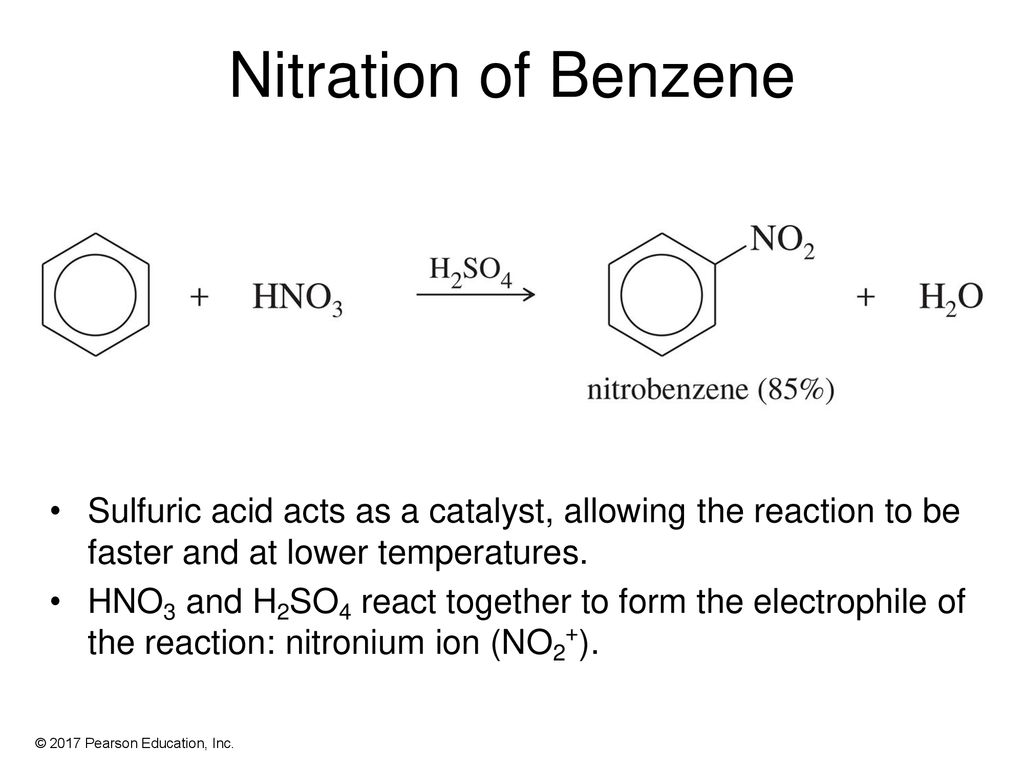
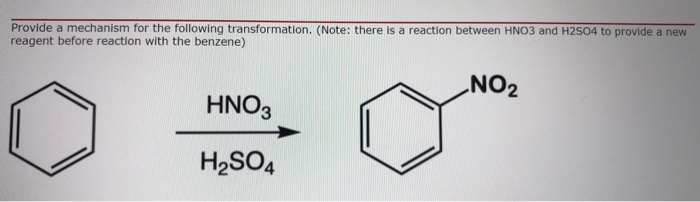
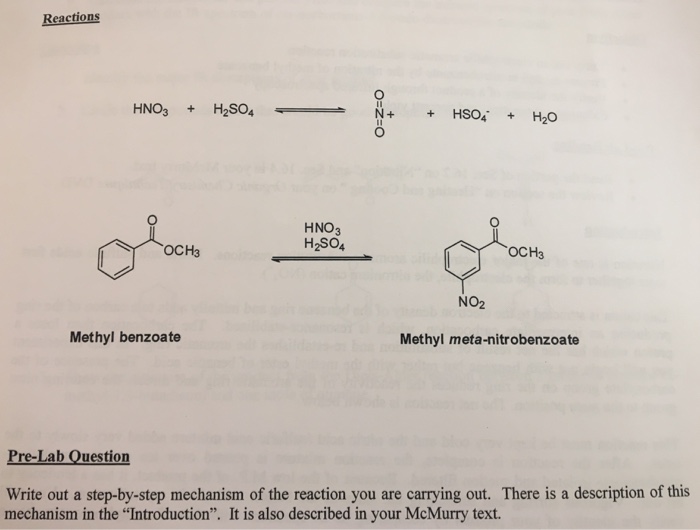
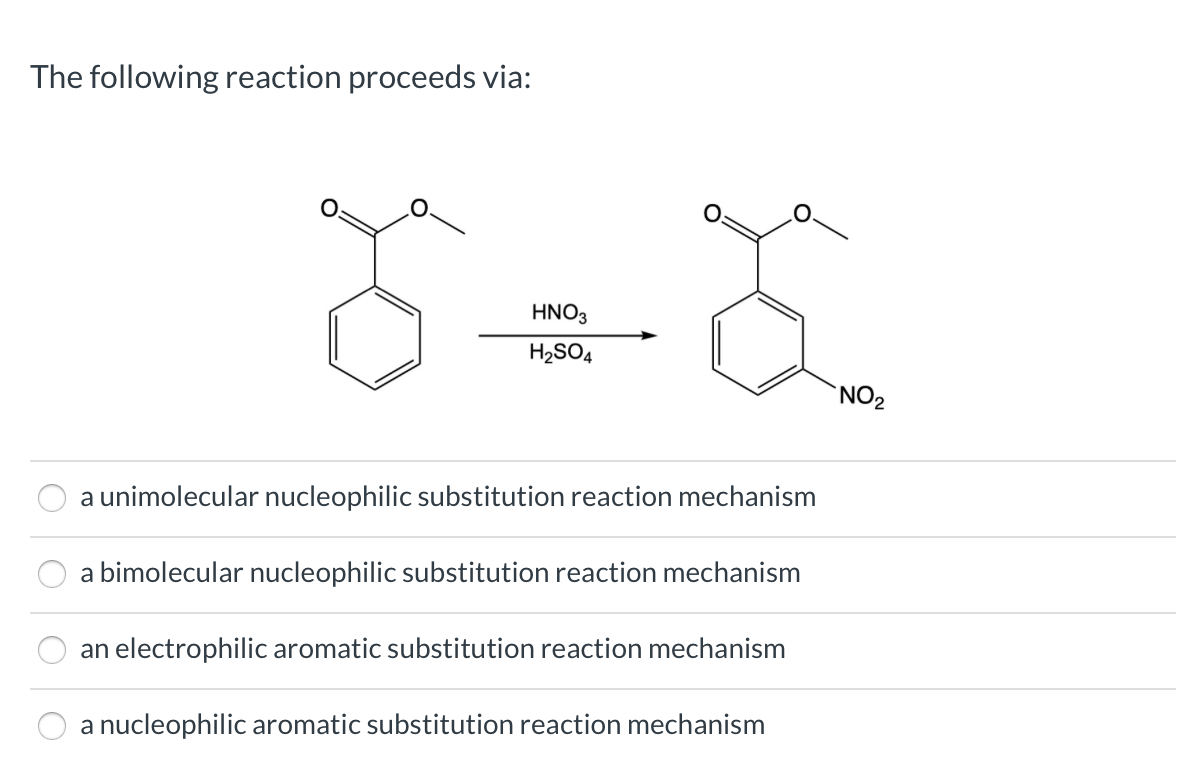
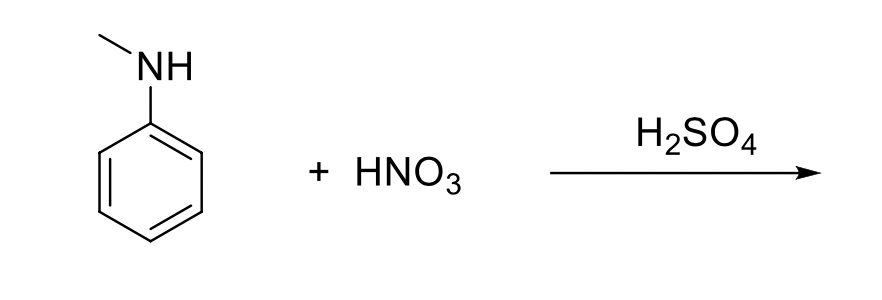Chemistry lovers - Nitration: A nitro group can be introduced into benzene by using a nitrating mixture to form nitro benzene. The nitrating mixture is a mixture of concentrated nitric acid and

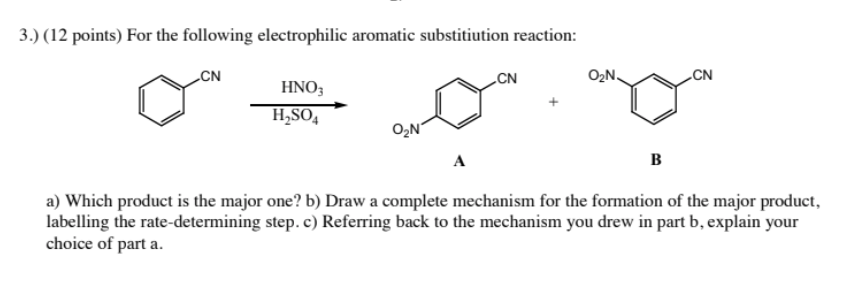
reaction mechanism - Nitration of tri-substituted benzene with Acetic acid and HNO3 - Chemistry Stack Exchange

benzene methylbenzene naphthalene mechanism nitration electrophilic substitution in benzene nitro-aromatic products reactions uses physical properties nitroaromatics nitroarenes nitrobenzene 1-methyl-2-nitrobenzene preparation advanced A level organic ...

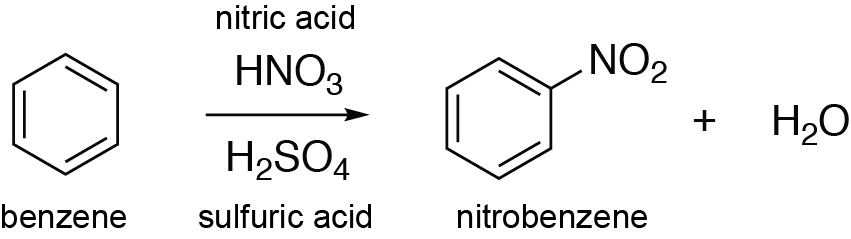
![Nitration [HNO3/H2SO4] - ChemistryScore Nitration [HNO3/H2SO4] - ChemistryScore](https://chemistryscore.com/wp-content/uploads/2019/11/Nitration2-1024x553.png)